Pregnant Women To Take Informed Decision On Vaccination; Govt Issues Guidance Note
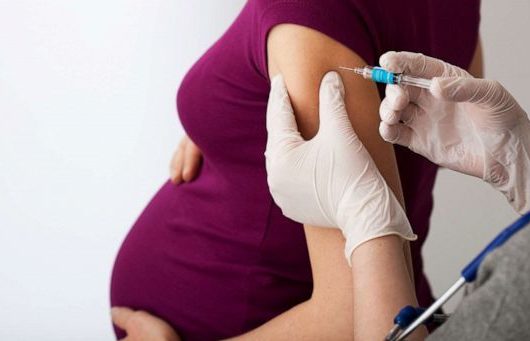
New Delhi: The Union Ministry of Health & Family Welfare on Thursday approved vaccination of pregnant women against COVID-19, based on the recommendations of National Technical Advisory Group on Immunization (NTAGI).
The approval is on condition that pregnant women be informed about the risks of exposure to COVID-19 infection along with risks and benefits associated with the vaccines available in the country.
The intent is to weigh risk versus benefit on individualized basis, so that a pregnant woman can take an informed decision. Based on the information provided, a pregnant woman will have the choice to take the vaccination.
A pregnant woman who opts for vaccination could be vaccinated at any time of the pregnancy.
The following guidance note enables states to develop a Counselling and Vaccination plan for pregnant women.
Preparing for COVID-19 vaccination of pregnant women
(i) Orientation and Capacity Building
Orientation of programme staff: States would undertake an orientation of programme managers responsible of the COVID vaccination programme at district, block and sub block levels, including Health and Wellness Centres and vaccinators at the health facilities in the public and private sector and also of all FLWs and health care providers at all the levels including medical colleges, DH, SDH, CHCs, PHCs, private clinics etc. who provide ANC services to women. The orientation would be conducted virtually and would broadly cover the areas in the attached sheet (Annexure l).
Training of FLWs and Vaccinations: From the above category, states would identify a pool of individuals (PHC-MOs, Staff Nurses, District Programme managers, and Community Health Officers etc. ) who would be directly be responsible for training of frontline workers and vaccinators on counseling of pregnant women and providing them with accurate information regarding the benefits and risks of the vaccine, including guiding them on registration and the location of the appropriate vaccination site. This training can be accomplished in two hours. It would need to be conducted in small batch (10-15) at the level of the PHC, ensuring COVID appropriate behaviors.
(ii) Engagement of medical professionals in the private sector
The state would conduct an orientation of members from professional bodies such as FOGSI, IMA, IAP & NNF, and any other state specific professional bodies they would be requested to ensure that this information is transmitted to all members. This could also be done virtually.
Counselling pregnant women for COVID Vaccination
There are several points at which interface of the pregnant woman and the FLW occurs and where pregnant women could be counselled. These include:
* Household visits by frontline workers
* Antenatal checkup at health facility, outreach immunization sessions, village health and nutrition days (VHNDs) and urban health and nutrition days (UHNDs).
* Facility visits by pregnant women for other reasons
* Any other site where there is interaction with the pregnant woman
* COVID-19 Vaccination Centers (CVCs).
During the counselling, the FLW or vaccinator (if the women reaches the CVC directly and has questions related to COVID 19 vaccination) should explain to the pregnant women the potential risks of COVID-19 on their health or that of the baby, benefits of vaccination, potential side effects and precautions they need to take following vaccination.
Vaccination of Pregnant Women
If the pregnant woman decides to get vaccinated, the process of registration for COVID-19 vaccination needs to be explained to her and the accompanying family member. She also needs to be informed about the nearest COVID vaccination centre, the modality for registration of beneficiaries, reporting of vaccination, generation of certificate etc. remains the same as general population.
Adverse Events following Vaccination
The full impact of COVID-19 disease on pregnancy outcomes for mother and fetus as well as for new-born is still unclear. Therefore, pregnant women require special considerations and systematic reporting of adverse events following immunization (AEFI). National AEFI surveillance operational guidelines and Covid-19 vaccination operational guidelines will be followed for AEFI surveillance related to Covid-19 vaccination of pregnant women.
During vaccination:
* The vaccinator or medical officer must consider the fact that women in reproductive age group might be unaware of the pregnancy at the time of vaccination. Therefore, the vaccinator must inform her for immediate reporting of AEFI, if any, following Covid-19 vaccination. In such cases, women will need to report immediately to the vaccinator or nearest health facility.
Reporting:
* The pregnancy status of women should be recorded into the AEFI notification form while reporting AEFI cases
* All Adverse Events following vaccination of pregnant women should be reported immediately into Co-WIN.
* All serious and severe adverse events following vaccination of pregnant women should be reported immediately to concerned Medical Officer / District Immunization Officer.
Investigation of cases:
* Obstetrician and gynecologist, pediatrician or neonatologist should be part of District AEFI Committee investigating all serious and severe AEFI cases following vaccination of pregnant women
* The investigation of all such cases to be expedited. Cytopathological examination of aborted/ perinatal death if any occurring in vaccinated women may be done
* The adverse event and the pregnancy outcome must be noted on the ANC/MCH card. Pregnancy registry can be used to track such cases and to determine pregnancy outcome.
* All antenatal, post-natal and other relevant clinical records must be sought for and collected during investigation and gathered from the treating physician.
Causality assessment:
* Causality assessment of all adverse events following Covid-19 vaccination of pregnant women
to be expedited.
Monitoring (as for current vaccination for COVID)
State Task forces (STF) will review planning, capacity building and implementation of pregnant women vaccination in the state.
District Task Forces (DTF)/Urban Task Forces (UTF) will be responsible for ensuring training of health workers, sensitization of professional bodies, and monitoring of vaccination activities for pregnant women.

Comments are closed.